Operationalizing RBQM Through Centralized Data Automation
The paradigm of Risk-Based Quality Management (RBQM) has shifted clinical trial oversight from exhaustive, uniform monitoring to a targeted, data-driven approach.
Guided by ICH E6(R3) [1], effective RBQM depends on Centralized Monitoring — the ability to identify and mitigate risks based on real-time data trends.
1. Understanding the Core Components of Risk-Based Monitoring
Risk-Based Quality Management (RBQM) in clinical trials takes a targeted, strategic approach that puts resources where they matter most - participant safety and data quality. Traditional monitoring approaches didn't account for site quality differences. RBQM brings together several connected parts that give a complete quality management system.
A solid RBQM strategy starts with risk assessment. Sponsors need to spot and group risks that could affect human subject protection and data integrity during protocol development. The original assessment looks at possible causes, detection likelihood, and what it all means for each identified risk. KRIs and QTLs each play their own role in the RBQM framework:
- Key Risk Indicators (KRIs) work as metrics that watch over critical data and study variables with set thresholds. Teams measure KRIs at the site level to guide site monitoring activities. They point out local operational risks and spark action when numbers cross thresholds.
- Quality Tolerance Limits (QTLs) are set parameters at the study level that catch system-wide issues that could affect subject safety or trial results. ICH E6(R3) brought in QTLs - the first time such limits became mandatory for good clinical practice.
2. The Challenge: Disparate Data and Delayed Risk Identification
Over the past decade, the amount of data collected per trial has increased by 283%[3], making comprehensive manual oversight nearly impossible. Most biotechs now apply RBQM in some form to detect and manage quality risks, yet true adoption remains a work in progress. Processes, teams, and technologies must continue to mature for RBQM to achieve consistent, measurable impact.
While RBQM mandates proactivity, execution often remains reactive due to data latency and manual processes. Most trial teams rely on periodic exports and manual integration across siloed systems to calculate Quality Tolerance Limits (QTLs) — predefined performance thresholds critical to trial reliability.
Typical Workflow Challenges:
- Data Scattered: Critical data (e.g., safety, lab results, protocol deviations, Imaging data) live in separate operational systems (EDC, Protocol Deviation (PD) Trackers, Central Lab, etc.).
- Manual Export & Integration: Data managers or central monitors manually export data sets on a fixed schedule (e.g., weekly or monthly).
- QTL Calculation: QTLs are calculated post-hoc, delaying visibility into emerging risks.
- Reactive Intervention: Investigation and corrective action begins, but valuable time is lost, increasing the risk of unrecoverable data loss or patient safety impact.
3. RIVIA's Automated Solution
RIVIA unifies and automates RBQM execution by continuously ingesting, harmonizing, and aggregating operational data streams - from EDC, PD trackers, Central Lab and imaging systems - into a single live view of the trial’s risk profile.
Unlike static reports, RIVIA enables real-time QTL and Key Risk Indicator (KRI) tracking, allowing central monitors to intervene before issues escalate.
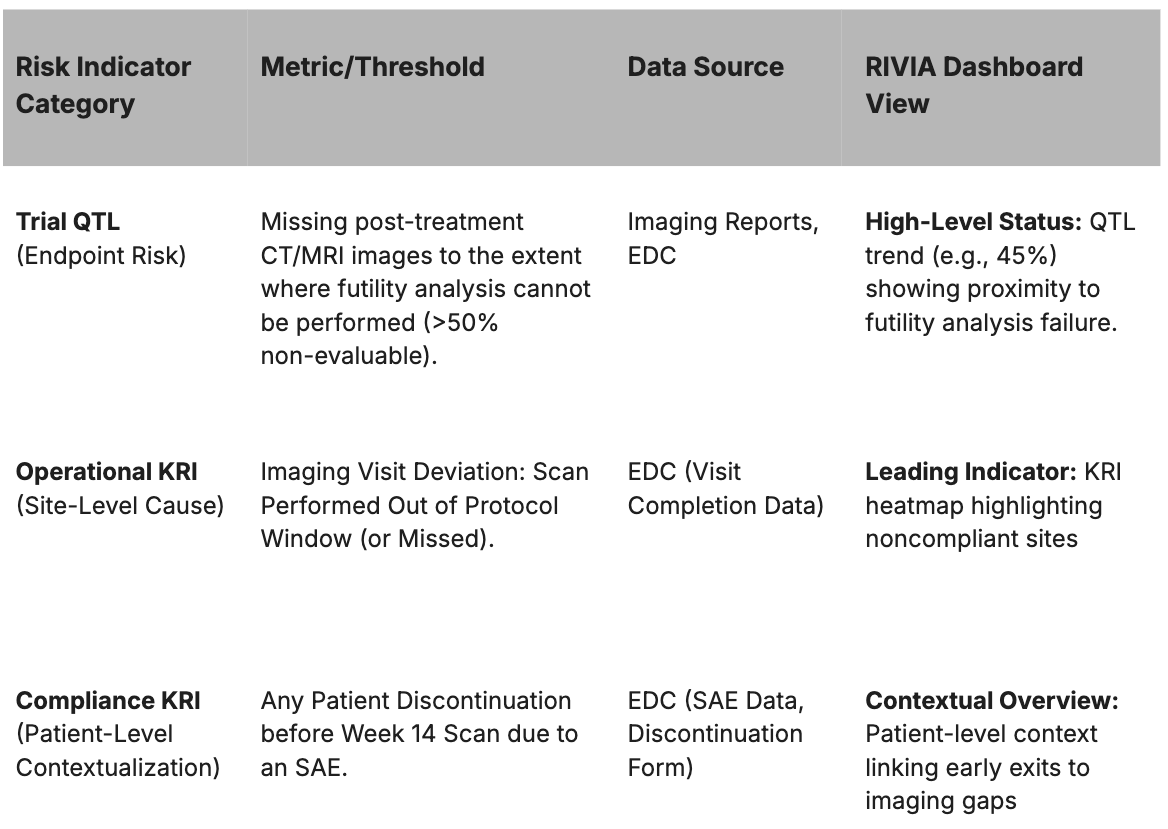
RIVIA's Proactive Workflow and Impact
The static RBQM plan is transformed into a dynamic monitoring system through a continuous, automated workflow:
- Continuous Data Integration: RIVIA automatically connects to and harmonizes operational data streams (EDC, Central Lab, Imaging) into a centralized live dashboard.
- Real-Time Limit Tracking: The live dashboard displays the continuous status of study-level Quality Tolerance Limits (QTLs) and site-level Key Risk Indicators (KRIs).
- Evidence-Based Decision-Making: When a KRI approaches a threshold, monitors can instantly drill down to patient-level records (SAEs, labs, deviations) to pinpoint root causes.
- Early Risk Mitigation: Teams intervene at the site or process level to address the root cause before accumulated errors result in a systemic QTL breach.
The resulting Operational and Regulatory Impact is significant: risk detection accelerates from weeks to hours, manual oversight burden is reduced, and the trial achieves stronger compliance with ICH E6(R3) principles for proactive quality assurance.
4. Conclusion
RIVIA operationalizes RBQM by unifying disparate trial data into a continuously monitored, automated system. Through real-time tracking of KRIs as leading indicators for overarching QTLs, RIVIA ensures risk control measures are applied promptly, proportionately, and intelligently - safeguarding data reliability, patient safety, and trial integrity.
References:
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. (2025, January 6). Guideline for good clinical practice E6(R3). Retrieved from https://www.ich.org/page/efficacy-guidelines
- U.S. Department of Health and Human Services, Food and Drug Administration. (2013, August). Guidance for industry: Oversight of clinical investigations—A risk-based approach to monitoring. Retrieved from https://www.fda.gov/media/116754/download
- Presentation: Uncovering RBQM Adoption Levels and Mapping Implementation: A Novel Comprehensive Assessment, A.Dirks, Tufts Center for the Study of Drug, Development, Presented at Precision in Clinical Trials Oncology, 18 Nov 2024, Boston USA.
Appendix
Regulatory and Evidence-Based Rationale: The use of a centralized data integration platform is directly supported by the principles of RBQM:
- Regulatory Mandate: ICH E6(R3) requires the establishment of QTLs to "identify systematic issues that can impact subject safety or reliability of trial results." These limits must be actively monitored and deviations must trigger a documented investigation and corrective action [1].
- Centralized Monitoring Efficacy: The FDA believes centralized monitoring can identify nearly 90% of on-site monitoring findings by focusing on data trends, anomalies, and systematic errors across sites (Result 2.1) [2].
- Proactive Mitigation: Effective RBQM necessitates identifying and addressing issues before they breach QTLs and compromise the trial. Data integration is the fundamental enabler of this proactivity [2].
Featured Testimonials
Read More
Latest News
.svg)



.png)

.svg)



